Periodic Table Groups Definition - Groups are the columns of the periodic table. The most common way the periodic table is classified by metals, nonmetals, and metalloids.
Periodic Table Electronegativity Trend Ionization
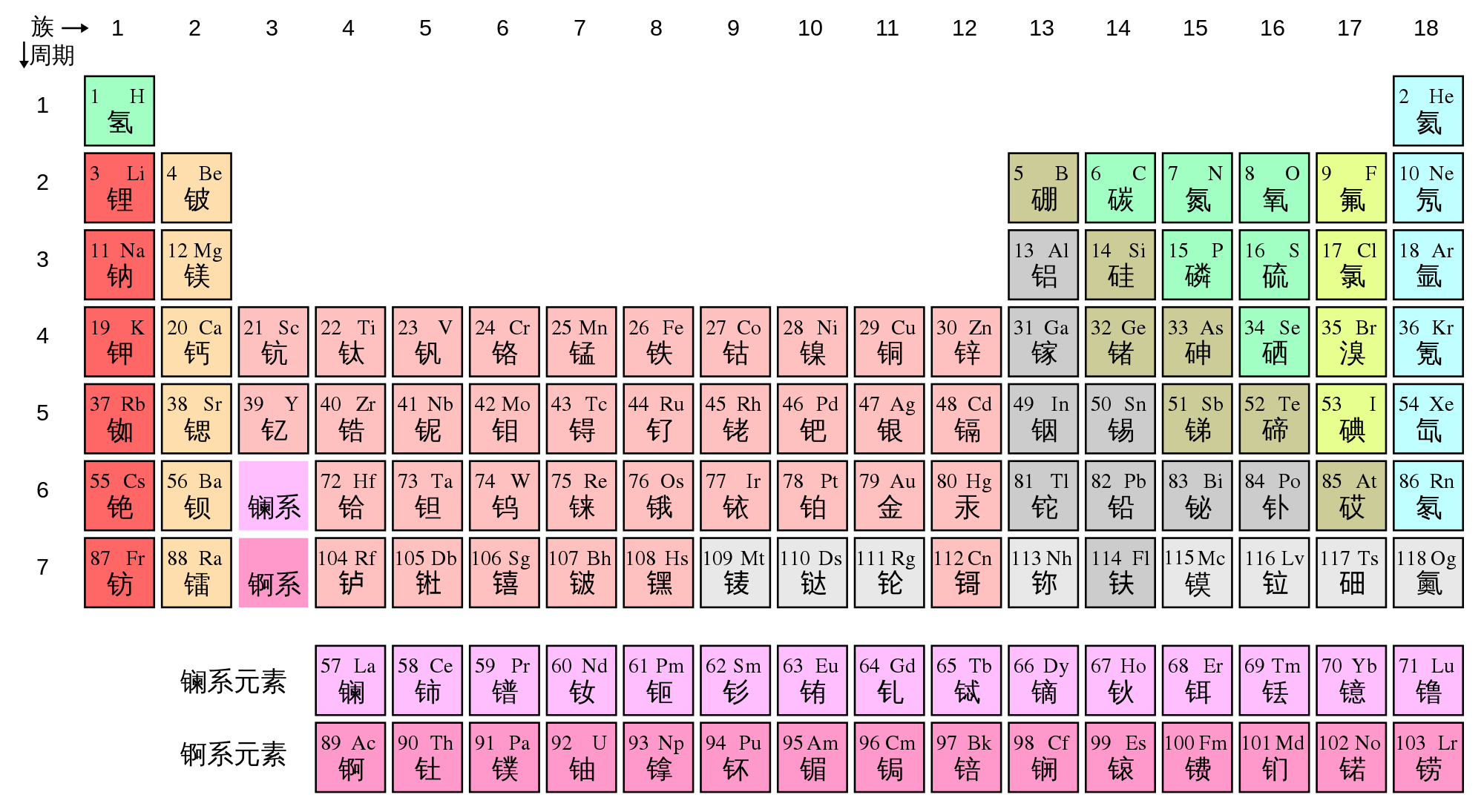
Elements with similar properties are arranged in the same column (called a group), and elements with the same number of electron shells are arranged in the same row (called a period).

Periodic table groups definition. Flip through this interactive periodic table of elements. Check out this free, online educational resource. The modern periodic table is based on the modern periodic law put forward by the english physicist henry moseley, which states that “the properties of.
There are multiple ways of grouping the elements, but they are commonly divided into metals, semimetals (metalloids), and nonmetals. Watch this brief video about the periodic table and element groups, from crash course. One example of a group is the noble or inert gases.
In chemistry, a group (also known as a family) is a vertical column in the periodic table of the chemical elements. Groups are numbered from 1 to 18. The american heritage® science dictionary copyright © 2011.
Periodic table groups are columns of elements found in the modern periodic table. There are 18 numbered groups in the periodic table; When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties.
In chemistry, a family is a group of elements with similar chemical properties.chemical families tend to be associated with the vertical columns on the periodic table.the term family is synonymous with the term group.because the two words have defined different sets of elements over the years, the iupac recommends the numerical system numbering elements from group 1 to group 18 be used. This video will explore the representative elements, focusing on each group or family. A periodic table is a tabular positioning of elements consisting of horizontal periods and vertical groups that helps in predicting the physical and chemical properties of the elements.
In a group, the chemical elements have atoms with identical valence electron counts and identical valence vacancy counts. These high definition images print and resize cleanly. Note you can find many more hd periodic tables in our printable table collection.click the link for the specific periodic table if you want a black background, other color options, or the pdf version of the table.
The atomic number of an element is the same as the number of protons in that particular nucleus of an atom.in the periodic table the elements are arranged into periods and groups. There are 18 columns or groups and different groups have different properties. These elements all line up in the eighteenth or last column of the periodic table.
Learn more about groups in this article. The same number may be assigned to d The periodic table is a way of arranging the elements so patterns in their properties and reactions can be identified and explained.
A group is also known as a family of atoms in which elements are arranged within each group of the periodic table. The number of each element corresponds to the number of protons in its nucleus (which is the same as the number of electrons orbiting that nucleus). The elements in each group have the same number of valence electrons.
A table of the elements, written in sequence in the order of atomic number or atomic weight and arranged in horizontal rows (periods) and vertical columns (groups) to illustrate the occurrence of similarities in the properties of the elements as a periodic function of the sequence. A table in which the chemical elements are arranged in order of increasing atomic number. This is what is meant by periodicity or periodic table trends.
The periodic table today is arranged with two different parts, the groups and the periods. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms, because most chemical properties are dominated by the orbital location of the outermost electron. The noble gases are very unreactive.
Periods in the periodic table in each period (horizontal row), the atomic numbers increase from left […] In the periodic table, the vertical columns are called ‘groups’ and the horizontal rows are called ‘periods’. There are three systems of group numbering for the groups;
18 vertical columns known as groups. The figure below shows the most commonly used form of the periodic table. A table in which the chemical elements are arranged in order of increasing atomic number.
In chemistry, a group is a column of elements in the periodic table of the chemical elements. The periodic table organizes elements into groups based on similar properties. The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical column or group.
Periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus. 7 horizontal rows known as periods. You'll find more specific groups, like transition metals, rare earths , alkali metals, alkaline earth, halogens, and noble gasses.
The chemical elements are the basic substances that make up all matter. The periodic table of the chemical elements is a list of known chemical elements.in the table, the elements are placed in the order of their atomic numbers starting with the lowest number of one, hydrogen. They all have a full outer shell of electrons, making them very stable (they tend not.
Elements with similar properties are arranged in the same column (called a group), and elements with the same number of electron shells are arranged in the same row (called a period). The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. The vertical columns of elements are called groups, or families.
As a result, elements in the same group often display similar properties and reactivity. The table is ‘periodic’ because elements with similar properties occur at regular intervals, i.e. There are a total of 18 groups.
In the periodic table of elements, there are seven horizontal rows of elements called periods. Therefore, the rows of the periodic table are called periods. This is a collection of periodic table of elements hd.
Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! Group, in chemistry, a column in the periodic table of the chemical elements. The periodic table, also known as the periodic table of elements, arranges the chemical elements such as hydrogen, silicon, iron, and uranium according to their recurring properties.
Elements in the same column have the same number of electrons in their outer shell (the highest energy level). The periodic table is a system for arranging the chemical elements. Groups are given a number to show where they are in the periodic table and also to identify the group of elements in them.
There are total 18 numbered groups in the modern periodic table, however, the “f” block columns between the group 2 and 3 are not numbered. the groups are the vertical columns. Mendeleev put elements with similar properties and that react in similar ways into the same.
Light metals these are elements of periodic table of group 1 and 2.
Pin by Zavalen Priodic on Table Priodic Sample Periodic
Main Group Elements Definition and Importance in 2020
Fresh Periodic Table Groups Properties Pdf tablepriodic
2000pxPeriodic_table_zhhans.svg.png (2000×1095)
Best Of Periodic Table Groups 3 12 Called (With images
Groups On the Periodic Table List Elements with Best Of
New Periodic Table Grade 7 Pdf Printable math worksheets
Giving Life The Periodic Table Relypsa, Inc. Periodic
New Periodic Table Groups and Families Periodic table
New Periodic Table Valence Shells Periodic table, Dmitri
New Periodic Table Groups Vs Periods Periodic table
Transition Metals Definition, List and Properties
New Periodic Table Groups and Families Periodic table
New Periodic Table Groups and Families Periodic table